Aim: To carry out the limit test for lead for the given sample
Apparatus & Chemical Requirements: Beaker, Glass rod, pipette, ammonium citrate, hydroxyl amine hydrochloride, phenol red, dil. Ammonia solution, potassium cyanide, Dithizone solution in chloroform, and standard lead nitrate.
Principle:
The limit test for lead is based upon the reaction between lead and diphenyl thiocarbazone or Dithiazone in chloroform is able to extract lead impurities from alkaline aqueous as a lead Dithizone complex which is red in color. The original Dithizone is having green color in chloroform while the lead Dihizone having violet or the red color. The intensity of color complex is depending upon on the amount of lead in solution. The color of lead Dithizone complex in chloroform is compared with the color produced by standard lead nitrate solution treated in a same manner.
Reaction:
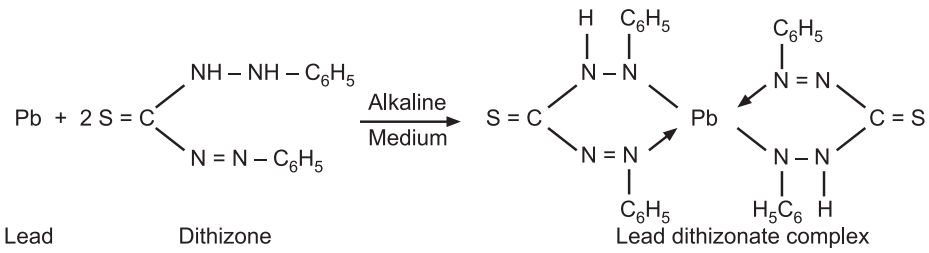
Procedure:
For sample- A specified amount of sample solution is prepared as directed in IP and taken in a separating funnel. 6ml of ammonium citrate, 2ml hydroxyl amine hydrochloride, 2 drops of phenol red is added. The solution is made alkaline by adding dil. ammonia solution and 2 ml of potassium cyanide is added. The alkaline solution is extracted with 5 ml portion of Dithizone solution in chloroform. Extraction is continued until the color of Dithizone layer remains green. The combined chloroform extract is shaken with 1% nitric acid, the Dithizone layer is taken into a beaker.
For standard- Specified quantity of lead nitrate is treated in the same manner as the sample solution.
Reasons:
- Reagent like hydroxyl amine hydrochloride, KCN, are added to prevent the interference of other impurities.
- Dil. Ammonia solution is added to make the solution alkaline which will be indicated by phenol red indicator, at this pH the extraction is optimum.

Post a Comment